Backed by 25 Years of Science and Research

What are stem cells?
Inside the human body are hundreds of different types of cells that fulfill specific tasks and are responsible for maintaining its everyday function. Among these are stem cells, responsible for tissue repair and regeneration (also known as tissue remodeling) to maintain a healthy body.
Stem cells can self-renew by dividing to generate more stem cells of the same type. They can also differentiate into specialized cell types. Scientific evidence shows mesenchymal stem cells are safe and effective for treating a wide range of diseases and conditions.
Scientific and Clinical Studies
- Scientific Presentations
- Our Patient-Reported Outcomes
- Published Research

At BioXcellerator, we use Wharton’s jelly-derived mesenchymal stem cells (WJ-MSCs) for our therapies.
Stem cells are adult/somatic cells that can be obtained from many different sources, such as bone marrow, adipose tissue, umbilical cords, and the placenta.
We use umbilical cord-derived mesenchymal stem cells, specifically from the Wharton’s jelly tissue (WJ-MSCs), as they are known to be among the most effective and have:
- Exhibit exceptional regenerative properties
- Highly effective anti-inflammatory properties—reducing levels of pro-inflammatory cytokines, increasing levels of anti-inflammatory cytokines, and secreting exosomes.
- Growth factors that the body can use to stimulate tissue repair and regeneration of muscles, tendons, ligaments, discs, cartilage, and other areas.
Allogeneic Therapies
These are allogeneic therapies, which means that our patients do not need to undergo extra invasive procedures for stem cell therapy. Our WJ-MSCs are sourced from donated umbilical cords. Umbilical cords are examined and analyzed in a clinical laboratory to ensure they are infection-free and are then processed in our cell bank to isolate and purify only mesenchymal stem cells.
Our lab complies with widely accepted international standards, and our protocols have been designed based on extensive research into selecting, testing, refining, and purifying cells before they are cultured and expanded into infusions with the highest potential to stimulate healing—our Signature Cells.
Screening
Although donors are screened for health issues, the cells extracted from umbilical cords are then tested for specific proteins and genes that research has identified offer high potential for effective treatment.
Purification
Cells that pass screening tests are purified and expanded so that therapy can be prepared that contains millions of high-potency cells that the body can use to promote healing, modulate the immune system, and reduce inflammation.
Ongoing Testing
Cells are tested as they are expanded and “passaged” into larger containers. Once expansion is complete, cells are tested again to verify that they meet various markers and criteria for purity and potency.
Cryo-Preservation
After cells have been expanded and tested, they are frozen in our cell bank. When reactivated, they are tested once more to ensure quality and viability.
What are the benefits of Wharton’s jelly umbilical cord-derived mesenchymal stem cells?
- Expression of typical mesenchymal markers and modest pluripotency genes, which may confer better efficacy with greater safety.
- In addition to the advantages of obtaining and expanding cells, WJ-MSCs have shown benefits in their immunomodulatory profile, showing that they have greater activity on lymphocytes through regulating the different cell populations.
- Due to the absence of certain surface proteins, the patient's immune system does not consider these cells as foreign, which reduces the likelihood of adverse reactions or graft vs. host disease.
Three mechanisms of how these cells can work are:
- Anti-inflammatory and angiogenesis through paracrine activity.
- Stabilize and repair damaged native cells through mitochondrial transfer and growth factors.
- Regenerate new tissue through exosome secretion to signal the body’s response to damage.
What are hypoxia-conditioned mesenchymal stem cells?
Preconditioning strategies to enhance the therapeutic function of MSCs in vitro include exposure to hypoxia, growth factors/cytokines, or conditioned medium.
Mesenchymal stem/stromal cells (MSCs) have been demonstrated to be promising cell sources for therapeutic stem cell therapy due to their capability of tissue regeneration and immunomodulation. MSCs also exert extensive paracrine effects through release of trophic factors and extracellular vesicles (EVs), like exosomes.
Hypoxia, or exposure to a low-oxygen environment, is a preconditioning protocol to improve the therapeutic potential of MSCs. Preconditioning strategies to improve the therapeutic function of MSCs in vitro include exposure to hypoxia, growth factors/cytokines, or conditioned medium.
Recent studies reported that MSCs cultured in a hypoxic environment may enhance the anti-inflammatory and migratory capacity of MSCs, improve angiogenic potency (new blood vessel formation), increase cell survival and proliferation, and reduce overall cell aging for improved protection and regeneration.
The enhanced therapeutic effects of hypoxia-cultured MSCs have been validated in many clinical studies. Hypoxia-preconditioned MSCs have been successfully used to regenerate various musculoskeletal tissues, including cartilage, bone, and tendon, after injury and halt the progression of diseases.

What are MSC exosomes?
Recent research on how stem cell therapy works gives scientists new insights that may help improve patient outcomes. Some studies demonstrate that adding exosomes to treatment protocols offers high therapeutic potential for some degenerative and age-related conditions, including chronic joint disease and skin aging.
Exosomes are naturally occurring extracellular vesicles in all types of cells, including mesenchymal stem cells (MSCs). They act as signaling agents, influencing other cells in the body by releasing:
- Anti-inflammatory cytokines
- Growth factors to promote healing
- Specific proteins that enhance cellular repair
Research demonstrates that not only does adding exosomes help enhance the repair of damage, but they also act quickly. This is why some patients receiving exosomes report more immediate relief of symptoms, and then continued improvement until they experience the full benefits of the MSCs they receive.
Exosomes are applied in the same manner and time as MSCs, so there are no additional procedures or injections required. At BioXcellerator, we isolate exosomes as we culture MSCs from umbilical cord tissue, and concentrate them at our on-site lab to provide the highest possible potency.
At BioXcellerator, we offer advanced protocols using both hypoxic and normoxic MSCs in our treatment protocols.
In both cases, we use Wharton’s jelly umbilical-cord derived mesenchymal stem cells (WJ-MSC). Research shows both the hypoxic and normoxic cells demonstrate exceptional anti-inflammatory properties, immune modulating capacity, and promote tissue repair and regeneration.
Precision Stem Cell Applications
MSC therapy has been studied worldwide for more than 50 years. Research demonstrates the safety and efficacy of mesenchymal stem cell treatments and their regenerative and anti-inflammatory capacity in pathologies such as:
- Arthritis and orthopedic injuries
- Neurologic diseases and disorders
- Dermatologic conditions
- Gastrointestinal disorders
- Urologic disorders
- Rheumatologic and autoimmune conditions
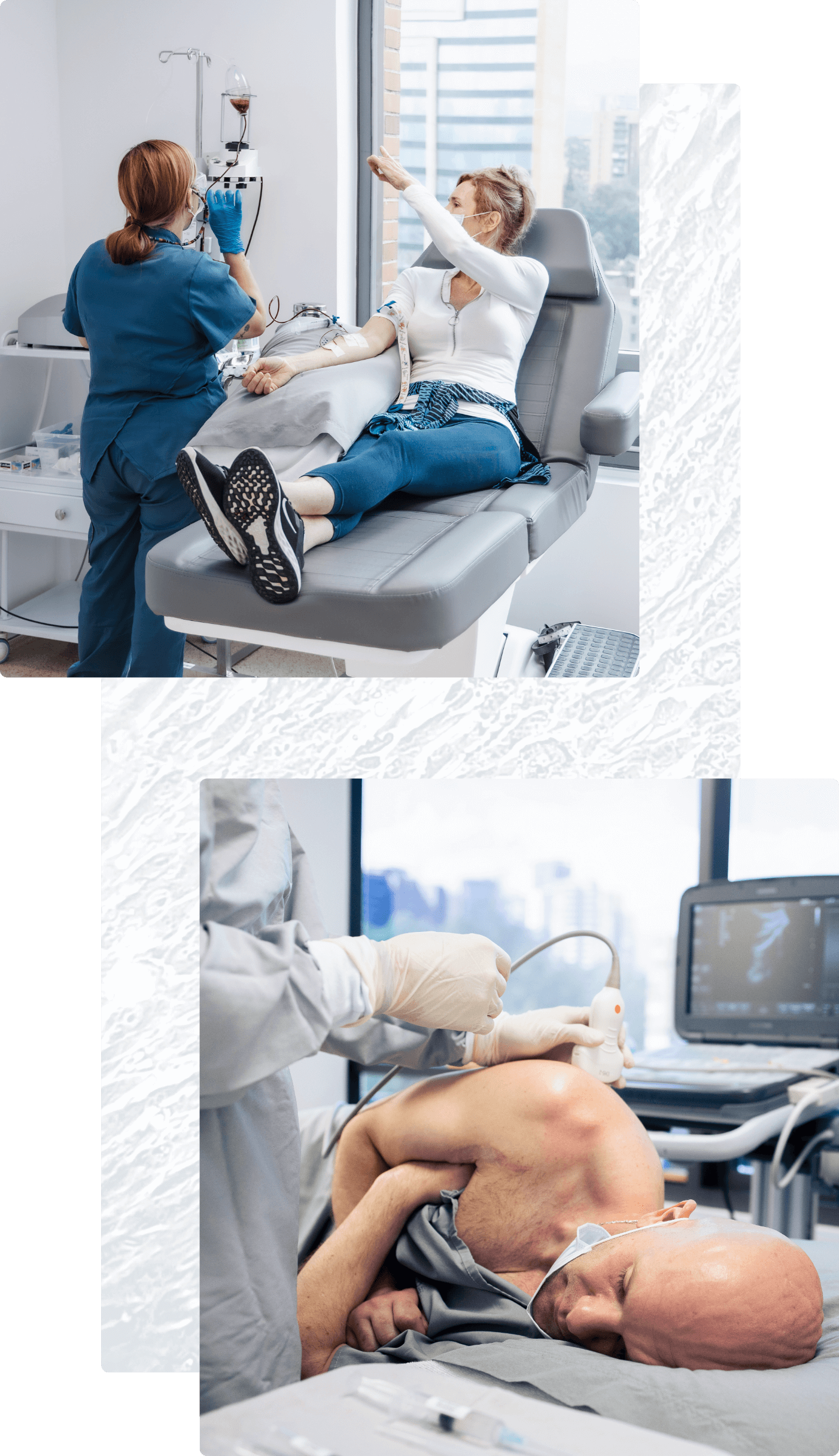
Most patient protocols include at least one intravenous (IV) WJ-MSC infusion. For this simple procedure, cells are infused intravenously to allow them to circulate throughout the body.
Other treatment protocols include direct injection at specific treatment sites. When appropriate, our physicians use advanced imaging technology such as X-ray fluoroscopy and diagnostic ultrasound to guide cells to these sites precisely. Depending on the condition being treated, these injections can include:
- Intralesional: Injection of cells directly into soft tissues.
- Intraarticular: Injection of cells directly into a joint, commonly used to treat arthritis or orthopedic injuries (does not require sedation).
- Intradiscal: Injection of cells into discs and facets of the spine (requires sedation).
- Intrathecal: Injection of cells directly into the spinal canal, usually requiring local anesthesia.
- Intradermal: Injection of cells directly into the dermal layer of the skin.
- Intracavernous: Application directly into the cavernous bodies of the penis.
- Intratesticular
- Intraovarian
- Intrauterine
- Intraprostatic
- Intracystic/bladder
Patient-Centered Care Model
Our medical and research teams collaborate to generate evidence that contributes significant information to the regenerative medicine, scientific, and academic communities.
Research and Innovation
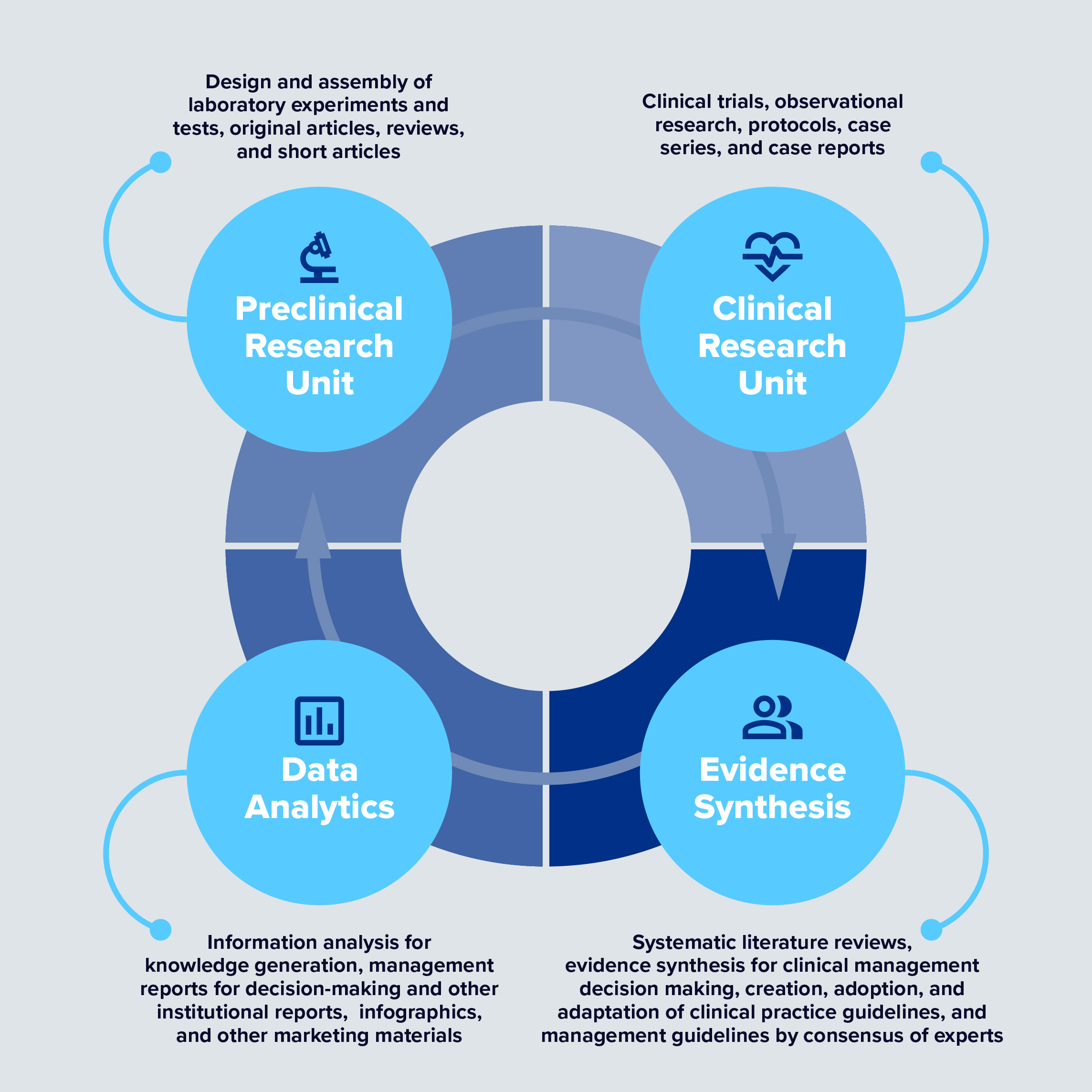
BioXscience, a subsidiary of BioXcellerator, is responsible for planning and executing our research and innovation activities aimed at identifying specific knowledge gaps in the field of regenerative medicine.
Our Methodology
The BioXscience team develops and publishes meta-analyses and systematic reviews to combine peer-reviewed literature, case studies, and observational data in the creation of best practice, patient-specific protocols that include MSC therapy with complementary therapies. Together, optimal patient outcomes are achieved with improvements in pain, function, and quality of life.
Our Evidence-Based Care Protocols
Our commitment to patient health is underscored by the rigorous integration of scientific evidence into our treatment protocols. We ensure that our treatment strategies meet the highest standards of scientific scrutiny based on global scientific evidence.
Our approach to healthcare is distinguished by evidence-based care protocols meticulously designed to address various medical conditions.
- Dermatological conditions
- Orthopedic/musculoskeletal disorders
- Degenerative disc and spine disorders
- Neurological disorders
- Rheumatologic and autoimmune diseases
- and other medical conditions
Our Outcomes
Our research results in the most innovative treatments and optimal patient outcomes in regenerative medicine:
- Development of the highest potency Wharton’s jelly umbilical cord MSCs
- The use of highly concentrated exosomes to complement MSC therapy
- Internationally recognized research publications and presentations
- 97% patient satisfaction after post-treatment survey
- Published patient-reported outcomes
References
- Schulman, C. I. et al. 354 Clinical Trial of Allogeneic Mesenchymal Stem Cells in Second Degree Burns: Prelim Results. J. Burn Care Res. 39, S147–S147 (2018).
- Fan, D. et al. Efficacy and safety of umbilical cord mesenchymal stem cells in the treatment of cesarean section skin scars: a randomized clinical trial. Stem Cell Res. Ther. 11, 244 (2020).
- Evaluation of the safety and efficacy of the treatment of scars and cutis laxa syndrome with the use of autologous (fresh and stored) stem cells isolated from adipose tissue within the project: The therapeutic potential of mesenchymal stem cells tested i. EudraCT number 2016-004110-10 (2021).
- Abo-Elkheir, W. et al. Role of cord blood and bone marrow mesenchymal stem cells in recent deep burn: A case-control prospective study. Am. J. Stem Cells 6, 23–35 (2017).
- Jiang, X., Zhang, H. & Teng, M. Effectiveness of Autologous Stem Cell Therapy for the Treatment of Lower Extremity Ulcers: A Systematic Review and Meta-Analysis. Medicine (Baltimore). 95, e2716 (2016).
- Shu, X. et al. The efficiency of stem cell based therapy in the treatment of diabetic foot ulcer: a meta-analysis. Endocr. J. 65, 403–413 (2018).
- Chiang, K. J., Chiu, L. C., Kang, Y. N. & Chen, C. Autologous Stem Cell Therapy for Chronic Lower Extremity Wounds: A Meta-Analysis of Randomized Controlled Trials. Cells 10, (2021).
- Zhang, C. et al. Topical and intravenous administration of human umbilical cord mesenchymal stem cells in patients with diabetic foot ulcer and peripheral arterial disease: a phase I pilot study with a 3-year follow-up. Stem Cell Res. Ther. 13, 451 (2022).
- Zhao, D. et al. Treatment of early stage osteonecrosis of the femoral head with autologous implantation of bone marrow-derived and cultured mesenchymal stem cells. Bone 50, 325–330 (2012).
- Aoyama, T. et al. An Exploratory Clinical Trial for Idiopathic Osteonecrosis of Femoral Head by Cultured Autologous Multipotent Mesenchymal Stromal Cells Augmented with Vascularized Bone Grafts. Tissue Eng. Part B Rev. 20, 233–242 (2014).
- Chen, C. et al. Efficacy of umbilical cord-derived mesenchymal stem cell-based therapy for osteonecrosis of the femoral head: A three-year follow-up study. Mol. Med. Rep. 14, 4209–4215 (2016).
- Wang, L., Tian, X., Li, K. & Liu, C. Combination use of core decompression for osteonecrosis of the femoral head: A systematic review and meta-analysis using Forest and Funnel Plots. Comput. Math. Methods Med. 2021, 1–10 (2021).
- Li, J., Su, P., Li, J., Chen, G. & Xiong, Y. Efficacy and Safety of Stem Cell Combination Therapy for Osteonecrosis of the Femoral Head: A Systematic Review and Meta-Analysis. J. Healthc. Eng. 2021, 1–8 (2021).
- Saw, K.-Y. et al. Articular Cartilage Regeneration With Autologous Peripheral Blood Stem Cells Versus Hyaluronic Acid: A Randomized Controlled Trial. Arthrosc. – J. Arthrosc. Relat. Surg. 29, 684–694 (2013).
- Skowroński, J. & Rutka, M. Osteochondral lesions of the knee reconstructed with mesenchymal stem cells – results. Ortop. Traumatol. Rehabil. 15, 1–1 (2013).
- Koh, Y.-G., Kwon, O.-R., Kim, Y.-S., Choi, Y.-J. & Tak, D.-H. Adipose-Derived Mesenchymal Stem Cells With Microfracture Versus Microfracture Alone: 2-Year Follow-up of a Prospective Randomized Trial. Arthrosc. – J. Arthrosc. Relat. Surg. 32, 97–109 (2016).
- de Windt, T. S. et al. Allogeneic Mesenchymal Stem Cells Stimulate Cartilage Regeneration and Are Safe for Single-Stage Cartilage Repair in Humans upon Mixture with Recycled Autologous Chondrons. Stem Cells 35, 256–264 (2017).
- de Windt, T. S. et al. Allogeneic MSCs and Recycled Autologous Chondrons Mixed in a One-Stage Cartilage Cell Transplantion: A First-in-Man Trial in 35 Patients. Stem Cells 35, 1984–1993 (2017).
- Hashimoto, Y. et al. Transplantation of autologous bone marrow-derived mesenchymal stem cells under arthroscopic surgery with microfracture versus microfracture alone for articular cartilage lesions in the knee: A multicenter prospective randomized control clinical trial. Regen. Ther. 11, 106–113 (2019).
- Cho, W. S. et al. Mesenchymal Stem Cells Use in the Treatment of Tendon Disorders: A Systematic Review and Meta-Analysis of Prospective Clinical Studies. Ann. Rehabil. Med. 45, 410–410 (2021).
- Havlas, V. et al. [Use of cultured human autologous bone marrow stem cells in repair of a rotator cuff tear: preliminary results of a safety study]. Acta Chir. Orthop. Traumatol. Cech. 82, 229–34 (2015).
- Lee, S. Y., Kim, W., Lim, C. & Chung, S. G. Treatment of Lateral Epicondylosis by Using Allogeneic Adipose-Derived Mesenchymal Stem Cells: A Pilot Study. Stem Cells 33, 2995–3005 (2015).
- Jo, C. H. et al. Intratendinous Injection of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells for the Treatment of Rotator Cuff Disease: A First-In-Human Trial. Stem Cells 36, 1441–1450 (2018).
- Lamas, J. R. et al. Adverse effects of xenogenic scaffolding in the context of a randomized double-blind placebo-controlled study for repairing full-thickness rotator cuff tears. Trials 20, 387 (2019).
- Mirghaderi, S. P. et al. Cell therapy efficacy and safety in treating tendon disorders: a systemic review of clinical studies. J. Exp. Orthop. 9, 85 (2022).
- Hernigou, P. et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: A case-controlled study. Int. Orthop. 38, 1811–1818 (2014).
- Centeno, C. J., Al-Sayegh, H., Bashir, J., Goodyear, S. H. & D Freeman, M. A prospective multi-site registry study of a specific protocol of autologous bone marrow concentrate for the treatment of shoulder rotator cuff tears and osteoarthritis. J. Pain Res. 8, 269–276 (2015).
- Kim, Y. S., Sung, C. H., Chung, S. H., Kwak, S. J. & Koh, Y. G. Does an Injection of Adipose-Derived Mesenchymal Stem Cells Loaded in Fibrin Glue Influence Rotator Cuff Repair Outcomes? A Clinical and Magnetic Resonance Imaging Study. Am. J. Sports Med. 45, 2010–2018 (2017).
- Jo, C. H., Chai, J. W., Jeong, E. C., Oh, S. & Yoon, K. S. Intratendinous Injection of Mesenchymal Stem Cells for the Treatment of Rotator Cuff Disease: A 2-Year Follow-Up Study. Arthrosc. – J. Arthrosc. Relat. Surg. 36, 971–980 (2020).
- Muench, L. N. et al. Preliminary Clinical Outcomes Following Biologic Augmentation of Arthroscopic Rotator Cuff Repair Using Subacromial Bursa, Concentrated Bone Marrow Aspirate, and Platelet-Rich Plasma. Arthrosc. Sport. Med. Rehabil. 2, e803–e813 (2020).
- Khoury, M. et al. Promising improvement of chronic lateral elbow tendinopathy by using adipose derived mesenchymal stromal cells: a pilot study. J. Exp. Orthop. 8, 6 (2021).
- Senesi, L. et al. Efficacy of Adipose-Derived Mesenchymal Stem Cells and Stromal Vascular Fraction Alone and Combined to Biomaterials in Tendinopathy or Tendon Injury: Systematic Review of Current Concepts. Medicina (B. Aires). 59, 273 (2023).
- Freitag, J., Shah, K., Wickham, J. & Tenen, A. Effect of autologous adipose-derived mesenchymal stem cell therapy in combination with autologous platelet-rich plasma in the treatment of elbow tendinopathy. BMJ Case Rep. 13, e234592 (2020).
- Kader, N. et al. Cell-based therapy in soft tissue sports injuries of the knee: a systematic review. Expert Opin. Biol. Ther. 21, 1035–1047 (2021).
- Olivos-Meza, A. et al. First Clinical Application of Polyurethane Meniscal Scaffolds with Mesenchymal Stem Cells and Assessment of Cartilage Quality with T2 Mapping at 12 Months. Cartilage 13, 197S-207S (2021).
- Vangsness, C. T. et al. Adult Human Mesenchymal Stem Cells Delivered via Intra-Articular Injection to the Knee Following Partial Medial Meniscectomy. J. Bone Jt. Surg. 96, 90–98 (2014).
- Agarwal, N., Mak, C., Bojanic, C., To, K. & Khan, W. Meta-Analysis of Adipose Tissue Derived Cell-Based Therapy for the Treatment of Knee Osteoarthritis. Cells 10, 1365 (2021).
- Doyle, E. C., Wragg, N. M. & Wilson, S. L. Intraarticular injection of bone marrow-derived mesenchymal stem cells enhances regeneration in knee osteoarthritis. Knee Surgery, Sport. Traumatol. Arthrosc. 28, 3827–3842 (2020).
- Emadedin, M. et al. Intra-articular injection of autologous mesenchymal stem cells in six patients with knee osteoarthritis. Arch. Iran. Med. 15, 422–428 (2012).
- Emadedin, M. et al. Intra-articular implantation of autologous bone marrow-derived mesenchymal stromal cells to treat knee osteoarthritis: a randomized, triple-blind, placebo-controlled phase 1/2 clinical trial. Cytotherapy 20, 1238–1246 (2018).
- Freitag, J. et al. Mesenchymal stem cell therapy in the treatment of osteoarthritis: Reparative pathways, safety and efficacy – A review. BMC Musculoskelet. Disord. 17, 1–13 (2016).
- Gupta, P. K. et al. Efficacy and safety of adult human bone marrow-derived, cultured, pooled, allogeneic mesenchymal stromal cells (Stempeucel®): preclinical and clinical trial in osteoarthritis of the knee joint. Arthritis Res. Ther. 18, 301 (2016).
- Ha, C.-W., Park, Y.-B., Kim, S. H. & Lee, H.-J. Intra-articular Mesenchymal Stem Cells in Osteoarthritis of the Knee: A Systematic Review of Clinical Outcomes and Evidence of Cartilage Repair. Arthrosc. – J. Arthrosc. Relat. Surg. 35, 277-288.e2 (2019).
- Haleem, A. M. et al. The clinical use of human culture-expanded autologous bone marrow mesenchymal stem cells transplanted on platelet-rich fibrin glue in the treatment of articular cartilage defects: A pilot study and preliminary results. Cartilage 1, 253–261 (2010).
- Han, X., Yang, B., Zou, F. & Sun, J. Clinical therapeutic efficacy of mesenchymal stem cells derived from adipose or bone marrow for knee osteoarthritis: A meta-analysis of randomized controlled trials. J. Comp. Eff. Res. 9, 361–374 (2020).
- Iijima, H., Isho, T., Kuroki, H., Takahashi, M. & Aoyama, T. Effectiveness of mesenchymal stem cells for treating patients with knee osteoarthritis: a meta-analysis toward the establishment of effective regenerative rehabilitation. npj Regen. Med. 3, (2018).
- Jevotovsky, D. S., Alfonso, A. R., Einhorn, T. A. & Chiu, E. S. Osteoarthritis and stem cell therapy in humans: a systematic review. Osteoarthr. Cartil. 26, 711–729 (2018).
- Al-Najar, M. et al. Intra-articular injection of expanded autologous bone marrow mesenchymal cells in moderate and severe knee osteoarthritis is safe: a phase I/II study. J. Orthop. Surg. Res. 12, 190 (2017).
- Jeyaraman, M., Muthu, S. & Ganie, P. A. Does the Source of Mesenchymal Stem Cell Have an Effect in the Management of Osteoarthritis of the Knee? Meta-Analysis of Randomized Controlled Trials. Cartilage 13, 1532S-1547S (2021).
- Jiang, P. et al. Efficacy and safety of mesenchymal stem cell injections for patients with osteoarthritis: a meta-analysis and review of RCTs. Arch. Orthop. Trauma Surg. 141, 1241–1251 (2021).
- Jo, C. H. et al. Intra-articular Injection of Mesenchymal Stem Cells for the Treatment of Osteoarthritis of the Knee: A 2-Year Follow-up Study. Am. J. Sports Med. 45, 2774–2783 (2017).
- Khalifeh Soltani, S. et al. Safety and efficacy of allogenic placental mesenchymal stem cells for treating knee osteoarthritis: a pilot study. Cytotherapy 21, 54–63 (2019).
- Kim, K.-I., Kim, M.-S. & Kim, J.-H. Intra-articular Injection of Autologous Adipose-Derived Stem Cells or Stromal Vascular Fractions: Are They Effective for Patients With Knee Osteoarthritis? A Systematic Review With Meta-analysis of Randomized Controlled Trials. Am. J. Sports Med. 51, 837–848 (2023).
- Kim, Y. S. et al. Assessment of clinical and MRI outcomes after mesenchymal stem cell implantation in patients with knee osteoarthritis: a prospective study. Osteoarthr. Cartil. 24, 237–245 (2016).
- Kim, Y. S., Choi, Y. J. & Koh, Y. G. Mesenchymal Stem Cell Implantation in Knee Osteoarthritis. Am. J. Sports Med. 43, 2293–2301 (2015).
- Kim, Y. S. et al. Mesenchymal Stem Cell Implantation in Osteoarthritic Knees. Am. J. Sports Med. 43, 176–185 (2015).
- Kim, Y. S. et al. Implantation of mesenchymal stem cells in combination with allogenic cartilage improves cartilage regeneration and clinical outcomes in patients with concomitant high tibial osteotomy. Knee Surgery, Sport. Traumatol. Arthrosc. 28, 544–554 (2020).
- Kim, Y. S. et al. Comparative Matched-Pair Analysis of the Injection Versus Implantation of Mesenchymal Stem Cells for Knee Osteoarthritis. Am. J. Sports Med. 43, 2738–2746 (2015).
- Álvarez Hernández, P. & de la Mata Llord, J. Expanded Mesenchymal Stromal Cells in knee osteoarthritis: A systematic literature review. Reumatol. Clínica (English Ed. 18, 49–55 (2022).
- Kim, Y. S., Suh, D. S., Tak, D. H., Chung, P. K. & Koh, Y. G. Mesenchymal Stem Cell Implantation in Knee Osteoarthritis: Midterm Outcomes and Survival Analysis in 467 Patients. Orthop. J. Sport. Med. 8, 232596712096918 (2020).
- Koh, Y.-G. et al. Mesenchymal Stem Cell Injections Improve Symptoms of Knee Osteoarthritis. Arthrosc. – J. Arthrosc. Relat. Surg. 29, 748–755 (2013).
- Koh, Y.-G., Kwon, O.-R., Kim, Y.-S. & Choi, Y.-J. Comparative Outcomes of Open-Wedge High Tibial Osteotomy With Platelet-Rich Plasma Alone or in Combination With Mesenchymal Stem Cell Treatment: A Prospective Study. Arthrosc. – J. Arthrosc. Relat. Surg. 30, 1453–1460 (2014).
- Koh, Y.-G. & Choi, Y.-J. Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee 19, 902–907 (2012).
- Kuah, D. et al. Safety, tolerability and efficacy of intra-articular Progenza in knee osteoarthritis: a randomized double-blind placebo-controlled single ascending dose study. J. Transl. Med. 16, 49 (2018).
- Lamo-Espinosa, J. M. et al. Phase II multicenter randomized controlled clinical trial on the efficacy of intra-articular injection of autologous bone marrow mesenchymal stem cells with platelet rich plasma for the treatment of knee osteoarthritis. J. Transl. Med. 18, 356 (2020).
- Lamo-Espinosa, J. M. et al. Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: long-term follow up of a multicenter randomized controlled clinical trial (phase I/II). J. Transl. Med. 16, 213 (2018).
- Lee, K. B. L., Wang, V. T. Z., Chan, Y. H. & Hui, J. H. P. A Novel, Minimally-Invasive Technique of Cartilage Repair in the Human Knee Using Arthroscopic Microfracture and Injections of Mesenchymal Stem Cells and Hyaluronic Acid—A Prospective Comparative Study on Safety and Short-Term Efficacy. Ann. Acad. Med. Singapore 41, 511–517 (2012).
- Lee, W.-S., Kim, H. J., Kim, K.-I., Kim, G. B. & Jin, W. Intra-Articular Injection of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis: A Phase IIb, Randomized, Placebo-Controlled Clinical Trial. Stem Cells Transl. Med. 8, 504–511 (2019).
- Liao, C.-D. et al. Comparative Efficacy of Intra-Articular Injection, Physical Therapy, and Combined Treatments on Pain, Function, and Sarcopenia Indices in Knee Osteoarthritis: A Network Meta-Analysis of Randomized Controlled Trials. Int. J. Mol. Sci. 24, 6078 (2023).
- Bastos, R. et al. Intra-articular injections of expanded mesenchymal stem cells with and without addition of platelet-rich plasma are safe and effective for knee osteoarthritis. Knee Surgery, Sport. Traumatol. Arthrosc. 26, 3342–3350 (2018).
- Lu, L. et al. Treatment of knee osteoarthritis with intra-articular injection of autologous adipose-derived mesenchymal progenitor cells: a prospective, randomized, double-blind, active-controlled, phase IIb clinical trial. Stem Cell Res. Ther. 10, 143 (2019).
- Lv, Z. et al. Advances in Mesenchymal Stem Cell Therapy for Osteoarthritis: From Preclinical and Clinical Perspectives. Bioengineering 10, 195 (2023).
- Ma, W. et al. Efficacy and safety of intra-articular injection of mesenchymal stem cells in the treatment of knee osteoarthritis. Medicine (Baltimore). 99, e23343 (2020).
- Matas, J. et al. Umbilical Cord-Derived Mesenchymal Stromal Cells (MSCs) for Knee Osteoarthritis: Repeated MSC Dosing Is Superior to a Single MSC Dose and to Hyaluronic Acid in a Controlled Randomized Phase I/II Trial. Stem Cells Transl. Med. 8, 215–224 (2019).
- McIntyre, J. A., Jones, I. A., Han, B. & Vangsness, C. T. Intra-articular Mesenchymal Stem Cell Therapy for the Human Joint: A Systematic Review. Am. J. Sports Med. 46, 3550–3563 (2018).
- Mehrabani, D. et al. The Healing Effect of Bone Marrow-Derived Stem Cells in Knee Osteoarthritis: A Case Report. World J. Plast. Surg. 5, 168–74 (2016).
- Moris, D. & Vernadakis, S. Renal Paratransplant Hernia. An Uncommon Variant of Internal Hernia. Are We Aware of It? Transplantation 97, e65–e66 (2014).
- Munar A., et al. Treatment of Knee Osteoarthritis with Autologous Expanded Bone Marrow Mesenchymal Stem Cells: 50 Cases Clinical and MRI Results at One Year Follow-Up. J. Stem Cell Res. Ther. 05, (2015).
- Nejadnik, H., Hui, J. H., Feng Choong, E. P., Tai, B.-C. & Lee, E. H. Autologous Bone Marrow–Derived Mesenchymal Stem Cells Versus Autologous Chondrocyte Implantation. Am. J. Sports Med. 38, 1110–1116 (2010).
- Orozco, L. et al. Treatment of Knee Osteoarthritis With Autologous Mesenchymal Stem Cells. Transplantation 95, 1535–1541 (2013).
- Biazzo, A., D’Ambrosi, R., Masia, F., Izzo, V. & Verde, F. Autologous adipose stem cell therapy for knee osteoarthritis: where are we now? Phys. Sportsmed. 48, 392–399 (2020).
- Pak, J. et al. Cartilage Regeneration in Humans with Adipose Tissue-Derived Stem Cells and Adipose Stromal Vascular Fraction Cells: Updated Status. Int. J. Mol. Sci. 19, 2146 (2018).
- Pintore, A. et al. Intra-articular injection of bone marrow aspirate concentrate (BMAC) or adipose-derived stem cells (ADSCs) for knee osteoarthritis: a prospective comparative clinical trial. J. Orthop. Surg. Res. 18, 350 (2023).
- Qu, H. & Sun, S. Efficacy of mesenchymal stromal cells for the treatment of knee osteoarthritis: a meta-analysis of randomized controlled trials. J. Orthop. Surg. Res. 16, 11 (2021).
- Shin, Y.-S., Yoon, J.-R., Kim, H.-S. & Lee, S.-H. Intra-Articular Injection of Bone Marrow-Derived Mesenchymal Stem Cells Leading to Better Clinical Outcomes without Difference in MRI Outcomes from Baseline in Patients with Knee Osteoarthritis. Knee Surg. Relat. Res. 30, 206–214 (2018).
- Soler, R. et al. Final results of a phase I–II trial using ex vivo expanded autologous Mesenchymal Stromal Cells for the treatment of osteoarthritis of the knee confirming safety and suggesting cartilage regeneration. Knee 23, 647–654 (2016).
- Song, Y. et al. Mesenchymal stem cells in knee osteoarthritis treatment: A systematic review and meta-analysis. J. Orthop. Transl. 24, 121–130 (2020).
- Song, Y. et al. Human adipose-derived mesenchymal stem cells for osteoarthritis: a pilot study with long-term follow-up and repeated injections. Regen. Med. 13, 295–307 (2018).
- Spasovski, D. et al. Intra‐articular injection of autologous adipose‐derived mesenchymal stem cells in the treatment of knee osteoarthritis. J. Gene Med. 20, (2018).
- Park, Y.-B., Ha, C.-W., Lee, C.-H., Yoon, Y. C. & Park, Y.-G. Cartilage Regeneration in Osteoarthritic Patients by a Composite of Allogeneic Umbilical Cord Blood-Derived Mesenchymal Stem Cells and Hyaluronate Hydrogel: Results from a Clinical Trial for Safety and Proof-of-Concept with 7 Years of Extended Follow-Up. Stem Cells Transl. Med. 6, 613–621 (2017).
- Tan, S. H. S. et al. Intra-articular Injections of Mesenchymal Stem Cells Without Adjuvant Therapies for Knee Osteoarthritis: A Systematic Review and Meta-analysis. Am. J. Sports Med. 49, 3113–3124 (2021).
- Chahal, J. et al. Bone Marrow Mesenchymal Stromal Cell Treatment in Patients with Osteoarthritis Results in Overall Improvement in Pain and Symptoms and Reduces Synovial Inflammation. Stem Cells Transl. Med. 8, 746–757 (2019).
- Toan, D. D. et al. The effectiveness of knee osteoarthritis treatment by arthroscopic microfracture technique in combination with autologous bone marrow stem cells transplantation. J. Back Musculoskelet. Rehabil. 33, 397–403 (2020).
- Pers, Y.-M. et al. Adipose Mesenchymal Stromal Cell-Based Therapy for Severe Osteoarthritis of the Knee: A Phase I Dose-Escalation Trial. Stem Cells Transl. Med. 5, 847–856 (2016).
- Jo, C. H. et al. Intra‐Articular Injection of Mesenchymal Stem Cells for the Treatment of Osteoarthritis of the Knee: A Proof‐of‐Concept Clinical Trial. Stem Cells 32, 1254–1266 (2014).
- Vega, A. et al. Treatment of Knee Osteoarthritis With Allogeneic Bone Marrow Mesenchymal Stem Cells. Transplantation 99, 1681–1690 (2015).
- Wakitani, S. et al. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthr. Cartil. 10, 199–206 (2002).
- Wiggers, T. G. H., Winters, M., Van den Boom, N. A., Haisma, H. J. & Moen, M. H. Autologous stem cell therapy in knee osteoarthritis: a systematic review of randomised controlled trials. Br. J. Sports Med. 55, 1161–1169 (2021).
- Wong, K. L. et al. Injectable Cultured Bone Marrow-Derived Mesenchymal Stem Cells in Varus Knees With Cartilage Defects Undergoing High Tibial Osteotomy: A Prospective, Randomized Controlled Clinical Trial With 2 Years’ Follow-up. Arthrosc. – J. Arthrosc. Relat. Surg. 29, 2020–2028 (2013).
- Xu, S. et al. Effect of mesenchymal stromal cells for articular cartilage degeneration treatment: a meta-analysis. Cytotherapy 17, 1342–1352 (2015).
- Yokota, N. et al. Comparative Clinical Outcomes After Intra-articular Injection With Adipose-Derived Cultured Stem Cells or Noncultured Stromal Vascular Fraction for the Treatment of Knee Osteoarthritis. Am. J. Sports Med. 47, 2577–2583 (2019).
- Yubo, M. et al. Clinical efficacy and safety of mesenchymal stem cell transplantation for osteoarthritis treatment: A meta-analysis. PLoS One 12, e0175449 (2017).
- Dai, W. et al. Intra-Articular Mesenchymal Stromal Cell Injections Are No Different From Placebo in the Treatment of Knee Osteoarthritis: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Arthrosc. – J. Arthrosc. Relat. Surg. 37, 340–358 (2021).
- Zhao, D. et al. Intra-Articular Injections of Platelet-Rich Plasma, Adipose Mesenchymal Stem Cells, and Bone Marrow Mesenchymal Stem Cells Associated With Better Outcomes Than Hyaluronic Acid and Saline in Knee Osteoarthritis: A Systematic Review and Network Meta-analysis. Arthrosc. – J. Arthrosc. Relat. Surg. 37, 2298-2314.e10 (2021).
- Freitag, J. et al. Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: a randomized controlled trial. Regen. Med. 14, 213–230 (2019).
- Wakitani, S. et al. Repair of articular cartilage defects in the patello-femoral joint with autologous bone marrow mesenchymal cell transplantation: three case reports involving nine defects in five knees. J. Tissue Eng. Regen. Med. 1, 74–79 (2007).
- Davatchi, F., Abdollahi, B. S., Mohyeddin, M., Shahram, F. & Nikbin, B. Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int. J. Rheum. Dis. 14, 211–215 (2011).
- Centeno, C. J. et al. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Physician 11, 343–53 (2008).
- Chengzhi, H. et al. Effect of platelet rich plasma combined with mesenchymal stem cells in treatment of knee osteoarthritis. Chinese J. Jt. Surgery (Electronic Version), 644–652 (2018).
- Zhang, S., Lyu, S., Ding, Q., Fan, M. & Tong, P. Intra-articular injection of autologous adipose-derived stem cells for knee osteoarthritis: a randomized controlled trial. Chinese J. Orthop. 12, 1426–1434 (2018).
- Wang, Y. et al. [Curative Effect of Human Umbilical Cord Mesenchymal Stem Cells by Intra-Articular Injection for Degenerative Knee Osteoarthritis]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 30, 1472–1477 (2016).
- Davatchi, F., Sadeghi Abdollahi, B., Mohyeddin, M. & Nikbin, B. Mesenchymal stem cell therapy for knee osteoarthritis: 5 years follow‐up of three patients. Int. J. Rheum. Dis. 19, 219–225 (2016).
- Ding, W. et al. Efficacy and Safety of Intra-Articular Cell-Based Therapy for Osteoarthritis: Systematic Review and Network Meta-Analysis. Cartilage 13, 104S-115S (2021).
- Pagotto, L. E. C., de Santana Santos, T. & Pastore, G. P. The efficacy of mesenchymal stem cells in regenerating structures associated with the temporomandibular joint: A systematic review. Arch. Oral Biol. 125, 105104 (2021).
- Chęciński, M. et al. Autologous Stem Cells Transplants in the Treatment of Temporomandibular Joints Disorders: A Systematic Review and Meta-Analysis of Clinical Trials. Cells 11, 2709 (2022).
- Liu, F., Meng, Q., Yin, H. & Yan, Z. Stem Cells in Rotator Cuff Injuries and Reconstructions: A Systematic Review and Meta-Analysis. Curr. Stem Cell Res. Ther. 14, 683–697 (2019).
- Aljabri, A. et al. The Safety and Efficacy of Stem Cell Therapy as an Emerging Therapy for ALS: A Systematic Review of Controlled Clinical Trials. Front. Neurol. 12, 1–7 (2021).
- Morata-Tarifa, C., Azkona, G., Glass, J., Mazzini, L. & Sanchez-Pernaute, R. Looking backward to move forward: a meta-analysis of stem cell therapy in amyotrophic lateral sclerosis. npj Regen. Med. 6, 20 (2021).
- Martinez, H. R. et al. Stem-cell transplantation into the frontal motor cortex in amyotrophic lateral sclerosis patients. Cytotherapy 11, 26–34 (2009).
- Rushkevich, Y. N. et al. The Use of Autologous Mesenchymal Stem Cells for Cell Therapy of Patients with Amyotrophic Lateral Sclerosis in Belarus. Bull. Exp. Biol. Med. 159, 576–581 (2015).
- Oh, K.-W. et al. Repeated Intrathecal Mesenchymal Stem Cells for Amyotrophic Lateral Sclerosis. Ann. Neurol. 84, 361–373 (2018).
- Yolcu, Y. U. et al. Use of regenerative treatments in treatment of lumbar Degenerative Disc Disease: A systematic review. Clin. Neurol. Neurosurg. 195, 105916 (2020).
- Xie, B. et al. Clinical Efficacy and Safety of Human Mesenchymal Stem Cell Therapy for Degenerative Disc Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Stem Cells Int. 2021, 1–9 (2021).
- Meisel, H.-J. et al. Cell Therapy for Treatment of Intervertebral Disc Degeneration: A Systematic Review. Glob. spine J. 9, 39S-52S (2019).
- Law, L. et al. Office-Based Mesenchymal Stem Cell Therapy for the Treatment of Musculoskeletal Disease: A Systematic Review of Recent Human Studies. Pain Med. 20, 1570–1583 (2019).
- Migliorini, F. et al. Autogenic mesenchymal stem cells for intervertebral disc regeneration. Int. Orthop. 43, 1027–1036 (2019).
- García de Frutos, A. et al. Randomized clinical trial: expanded autologous bone marrow mesenchymal cells combined with allogeneic bone tissue, compared with autologous iliac crest graft in lumbar fusion surgery. Spine J. 20, 1899–1910 (2020).
- Amirdelfan, K. et al. Allogeneic mesenchymal precursor cells treatment for chronic low back pain associated with degenerative disc disease: a prospective randomized, placebo-controlled 36-month study of safety and efficacy. Spine J. 21, 212–230 (2021).
- Baroncini, A. et al. Mesenchymal Stem Cell Applications in Spine Disorders: A Comprehensive Review. Appl. Sci. 11, 7966 (2021).
- Miranda, L., Quaranta, M., Oliva, F. & Maffulli, N. Stem cells and discogenic back pain. Br. Med. Bull. 146, 73–87 (2023).
- Noriega, D. C. et al. Intervertebral Disc Repair by Allogeneic Mesenchymal Bone Marrow Cells: A Randomized Controlled Trial. Transplantation 101, 1945–1951 (2017).
- Oehme, D., Goldschlager, T., Ghosh, P., Rosenfeld, J. V. & Jenkin, G. Cell-Based Therapies Used to Treat Lumbar Degenerative Disc Disease: A Systematic Review of Animal Studies and Human Clinical Trials. Stem Cells Int. 2015, 1–16 (2015).
- Orozco, L. et al. Intervertebral Disc Repair by Autologous Mesenchymal Bone Marrow Cells: A Pilot Study. Transplantation 92, 822–828 (2011).
- Pang, X., Yang, H. & Peng, B. Human umbilical cord mesenchymal stem cell transplantation for the treatment of chronic discogenic low back pain. Pain Physician 17, E525-30 (2014).
- Sakai, D. & Andersson, G. B. J. Stem cell therapy for intervertebral disc regeneration: obstacles and solutions. Nat. Rev. Rheumatol. 11, 243–256 (2015).
- Sanapati, J. et al. Do Regenerative Medicine Therapies Provide Long-Term Relief in Chronic Low Back Pain: A Systematic Review and Metaanalysis. Pain Physician 21, 515–540 (2018).
- Soufi, K. H., Castillo, J. A., Rogdriguez, F. Y., DeMesa, C. J. & Ebinu, J. O. Potential Role for Stem Cell Regenerative Therapy as a Treatment for Degenerative Disc Disease and Low Back Pain: A Systematic Review. Int. J. Mol. Sci. 24, 8893 (2023).
- Yoshikawa, T., Ueda, Y., Miyazaki, K., Koizumi, M. & Takakura, Y. Disc Regeneration Therapy Using Marrow Mesenchymal Cell Transplantation. Spine (Phila. Pa. 1976). 35, E475–E480 (2010).
- Zhang, W. et al. Application of stem cells in the repair of intervertebral disc degeneration. Stem Cell Res. Ther. 13, 70 (2022).
- Centeno, C. et al. Treatment of lumbar degenerative disc disease-associated radicular pain with culture-expanded autologous mesenchymal stem cells: a pilot study on safety and efficacy. J. Transl. Med. 15, 197 (2017).
- Desai, M. J., Mansfield, J. T., Robinson, D. M., Miller, B. C. & Borg‐Stein, J. Regenerative Medicine for Axial and Radicular Spine‐Related Pain: A Narrative Review. Pain Pract. 20, 437–453 (2020).
- Elabd, C. et al. Intra-discal injection of autologous, hypoxic cultured bone marrow-derived mesenchymal stem cells in five patients with chronic lower back pain: a long-term safety and feasibility study. J. Transl. Med. 14, 253 (2016).
- El-Kadiry, A. E.-H., Lumbao, C., Rafei, M. & Shammaa, R. Autologous BMAC Therapy Improves Spinal Degenerative Joint Disease in Lower Back Pain Patients. Front. Med. 8, 1–11 (2021).
- Henriksson, H. B. et al. The Traceability of Mesenchymal Stromal Cells After Injection Into Degenerated Discs in Patients with Low Back Pain. Stem Cells Dev. 28, 1203–1211 (2019).
- Hunt, C. L. et al. Current understanding of safety and efficacy of stem cell therapy for discogenic pain—A systematic review of human studies. Tech. Reg. Anesth. Pain Manag. 19, 32–37 (2015).
- Kumar, H. et al. Safety and tolerability of intradiscal implantation of combined autologous adipose-derived mesenchymal stem cells and hyaluronic acid in patients with chronic discogenic low back pain: 1-year follow-up of a phase I study. Stem Cell Res. Ther. 8, 262 (2017).
- Lewandrowski, K.-U. et al. Pain Relief After Allogenic Stem Cell Disc Therapy. Pain Physician 26, 197–206 (2023).
- Kim, H. J. et al. Stereotactic brain injection of human umbilical cord blood mesenchymal stem cells in patients with Alzheimer’s disease dementia: A phase 1 clinical trial. Alzheimer’s Dement. Transl. Res. Clin. Interv. 1, 95–102 (2015).
- Lukashev, A. et al. Stem cells for Alzheimer’s disease: Safety in clinical trials. Alzheimer’s Dement. 12, P618 (2016).
- Kim, H. J. et al. Intracerebroventricular injection of human umbilical cord blood mesenchymal stem cells in patients with Alzheimer’s disease dementia: a phase I clinical trial. Alzheimers. Res. Ther. 13, 154 (2021).
- Brody, M. et al. Results and insights from a phase I clinical trial of Lomecel‐B for Alzheimer’s disease. Alzheimer’s Dement. 19, 261–273 (2023).
- Hlebokazov, F. et al. Treatment of refractory epilepsy patients with autologous mesenchymal stem cells reduces seizure frequency: An open label study. Adv. Med. Sci. 62, 273–279 (2017).
- Hlebokazov, F. et al. Clinical benefits of single vs repeated courses of mesenchymal stem cell therapy in epilepsy patients. Clin. Neurol. Neurosurg. 207, 106736 (2021).
- Aligholi, H., Safahani, M. & Asadi-Pooya, A. A. Stem cell therapy in patients with epilepsy: A systematic review. Clin. Neurol. Neurosurg. 200, 106416 (2021).
- Ramos‐Fresnedo, A. et al. Mesenchymal stem cell therapy for focal epilepsy: A systematic review of preclinical models and clinical studies. Epilepsia 63, 1607–1618 (2022).
- Berard, J. A. et al. Mesenchymal stem cell therapy and cognition in MS: Preliminary findings from a phase II clinical trial. Mult. Scler. Relat. Disord. 61, 103779 (2022).
- Tremblay, F., Ansari, Y., Remaud, A. & Freedman, M. S. Neurophysiological outcomes following mesenchymal stem cell therapy in multiple sclerosis. Clin. Neurophysiol. 136, 69–81 (2022).
- Riordan, N. H. et al. Clinical feasibility of umbilical cord tissue-derived mesenchymal stem cells in the treatment of multiple sclerosis. J. Transl. Med. 16, 57 (2018).
- Uccelli, A. et al. Safety, tolerability, and activity of mesenchymal stem cells versus placebo in multiple sclerosis (MESEMS): a phase 2, randomised, double-blind crossover trial. Lancet Neurol. 20, 917–929 (2021).
- Uccelli, A. et al. Mesenchymal Stem cells for Multiple Sclerosis (MESEMS): a randomized, double blind, cross-over phase I/II clinical trial with autologous mesenchymal stem cells for the therapy of multiple sclerosis. Trials 20, 263 (2019).
- Yamout, B. et al. Bone marrow mesenchymal stem cell transplantation in patients with multiple sclerosis: A pilot study. J. Neuroimmunol. 227, 185–189 (2010).
- Fernández, O. et al. Adipose-derived mesenchymal stem cells (AdMSC) for the treatment of secondary-progressive multiple sclerosis: A triple blinded, placebo controlled, randomized phase I/II safety and feasibility study. PLoS One 13, e0195891 (2018).
- Karussis, D. et al. Safety and Immunological Effects of Mesenchymal Stem Cell Transplantation in Patients With Multiple Sclerosis and Amyotrophic Lateral Sclerosis. Arch. Neurol. 67, 1187–1194 (2010).
- Li, J.-F. et al. The Potential of Human Umbilical Cord-Derived Mesenchymal Stem Cells as a Novel Cellular Therapy for Multiple Sclerosis. Cell Transplant. 23, 113–122 (2014).
- Llufriu, S. et al. Randomized Placebo-Controlled Phase II Trial of Autologous Mesenchymal Stem Cells in Multiple Sclerosis. PLoS One 9, e113936 (2014).
- Lublin, F. D. et al. Human placenta-derived cells (PDA-001) for the treatment of adults with multiple sclerosis: A randomized, placebo-controlled, multiple-dose study. Mult. Scler. Relat. Disord. 3, 696–704 (2014).
- Meng, M. et al. Umbilical cord mesenchymal stem cell transplantation in the treatment of multiple sclerosis. Am. J. Transl. Res. 10, 212–223 (2018).
- Petrou, P. et al. Beneficial effects of autologous mesenchymal stem cell transplantation in active progressive multiple sclerosis. Brain 143, 3574–3588 (2020).
- Petrou, P. et al. Long-Term Clinical and Immunological Effects of Repeated Mesenchymal Stem Cell Injections in Patients With Progressive Forms of Multiple Sclerosis. Front. Neurol. 12, 1–13 (2021).
- Lu, Z. et al. Human Umbilical Cord Mesenchymal Stem Cell Therapy on Neuromyelitis Optica. Curr. Neurovasc. Res. 9, 250–255 (2012).
- Yao, X.-Y. et al. Human Umbilical Cord Mesenchymal Stem Cells to Treat Neuromyelitis Optica Spectrum Disorder (hUC–MSC–NMOSD): A Study Protocol for a Prospective, Multicenter, Randomized, Placebo-Controlled Clinical Trial. Front. Neurol. 13, 1–10 (2022).
- Vaquero, J. et al. Repeated subarachnoid administrations of autologous mesenchymal stromal cells supported in autologous plasma improve quality of life in patients suffering incomplete spinal cord injury. Cytotherapy 19, 349–359 (2017).
- Vaquero, J. et al. Intrathecal administration of autologous mesenchymal stromal cells for spinal cord injury: Safety and efficacy of the 100/3 guideline. Cytotherapy 20, 806–819 (2018).
- Vaquero, J. et al. Cell therapy with autologous mesenchymal stromal cells in post-traumatic syringomyelia. Cytotherapy 20, 796–805 (2018).
- Gibbons, C. H. et al. Phase 2a randomized controlled study investigating the safety and efficacy of PDA‐002 in diabetic peripheral neuropathy. J. Peripher. Nerv. Syst. 26, 276–289 (2021).
- Abdelaziz, O. S., Marie, A., Abbas, M., Ibrahim, M. & Gabr, H. Feasibility, safety, and efficacy of directly transplanting autologous adult bone marrow stem cells in patients with chronic traumatic dorsal cord injury: A pilot clinical study. Neurosurg. Q. 20, 216–226 (2010).
- Honmou, O. et al. Intravenous infusion of auto serum-expanded autologous mesenchymal stem cells in spinal cord injury patients: 13 case series. Clin. Neurol. Neurosurg. 203, 106565 (2021).
- Hur, J. W. et al. Intrathecal transplantation of autologous adipose-derived mesenchymal stem cells for treating spinal cord injury: A human trial. J. Spinal Cord Med. 39, 655–664 (2016).
- Jeon, S. R. et al. Treatment of spinal cord injury with bone marrow-derived, cultured autologous mesenchymal stem cells. Tissue Engineering and Regenerative Medicine vol. 7 316–322 (2010).
- Jiang, P.-C. et al. A clinical trial report of autologous bone marrow-derived mesenchymal stem cell transplantation in patients with spinal cord injury. Exp. Ther. Med. 6, 140–146 (2013).
- Karamouzian, S., Nematollahi-Mahani, S. N., Nakhaee, N. & Eskandary, H. Clinical safety and primary efficacy of bone marrow mesenchymal cell transplantation in subacute spinal cord injured patients. Clin. Neurol. Neurosurg. 114, 935–939 (2012).
- Kishk, N. A. et al. Case Control Series of Intrathecal Autologous Bone Marrow Mesenchymal Stem Cell Therapy for Chronic Spinal Cord Injury. Neurorehabil. Neural Repair 24, 702–708 (2010).
- Liu, J. et al. Clinical analysis of the treatment of spinal cord injury with umbilical cord mesenchymal stem cells. Cytotherapy 15, 185–191 (2013).
- Liu, S. et al. A Comparative Study of Different Stem Cell Transplantation for Spinal Cord Injury: A Systematic Review and Network Meta-Analysis. World Neurosurg. 159, e232–e243 (2022).
- Mendonça, M. V. P. et al. Safety and neurological assessments after autologous transplantation of bone marrow mesenchymal stem cells in subjects with chronic spinal cord injury. Stem Cell Res. Ther. 5, 126 (2014).
- Muthu, S., Jeyaraman, M., Gulati, A. & Arora, A. Current evidence on mesenchymal stem cell therapy for traumatic spinal cord injury: systematic review and meta-analysis. Cytotherapy 23, 186–197 (2021).
- Ahuja, C. S. et al. The leading edge: Emerging neuroprotective and neuroregenerative cell-based therapies for spinal cord injury. Stem Cells Transl. Med. 9, 1509–1530 (2020).
- Oh, S. K. et al. A Phase III Clinical Trial Showing Limited Efficacy of Autologous Mesenchymal Stem Cell Therapy for Spinal Cord Injury. Neurosurgery 78, 436–447 (2016).
- Pal, R. et al. Ex vivo-expanded autologous bone marrow-derived mesenchymal stromal cells in human spinal cord injury/paraplegia: a pilot clinical study. Cytotherapy 11, 897–911 (2009).
- Park, H. C. et al. Treatment of Complete Spinal Cord Injury Patients by Autologous Bone Marrow Cell Transplantation and Administration of Granulocyte-Macrophage Colony Stimulating Factor. Tissue Eng. 11, 913–922 (2005).
- Park, J. H. et al. Long-term Results of Spinal Cord Injury Therapy Using Mesenchymal Stem Cells Derived From Bone Marrow in Humans. Neurosurgery 70, 1238–1247 (2012).
- Saini, R. et al. Efficacy and outcome of bone marrow derived stem cells transplanted via intramedullary route in acute complete spinal cord injury – A randomized placebo controlled trial. J. Clin. Neurosci. 100, 7–14 (2022).
- Saito, F. et al. Administration of cultured autologous bone marrow stromal cells into cerebrospinal fluid in spinal injury patients: A pilot study. Restor. Neurol. Neurosci. 30, 127–136 (2012).
- Satti, H. S. et al. Autologous mesenchymal stromal cell transplantation for spinal cord injury: A Phase I pilot study. Cytotherapy 18, 518–522 (2016).
- Shang, Z., Wang, M., Zhang, B., Wang, X. & Wanyan, P. Clinical translation of stem cell therapy for spinal cord injury still premature: results from a single-arm meta-analysis based on 62 clinical trials. BMC Med. 20, 284 (2022).
- Silvestro, S., Bramanti, P., Trubiani, O. & Mazzon, E. Stem Cells Therapy for Spinal Cord Injury: An Overview of Clinical Trials. Int. J. Mol. Sci. 21, 659 (2020).
- Song, H. et al. Bone Marrow Mesenchymal Stem Cells Transplantation on Acute Spinal Cord Injury. J. Hard Tissue Biol. 29, 91–98 (2020).
- Albu, S. et al. Clinical effects of intrathecal administration of expanded Wharton jelly mesenchymal stromal cells in patients with chronic complete spinal cord injury: a randomized controlled study. Cytotherapy 23, 146–156 (2021).
- Tang, Q.-R. et al. Evaluation of the Clinical Efficacy of Stem Cell Transplantation in the Treatment of Spinal Cord Injury: A Systematic Review and Meta-analysis. Cell Transplant. 30, 096368972110678 (2021).
- Tien, N. L. B. et al. Autologous Transplantation of Adipose-Derived Stem Cells to Treat Acute Spinal Cord Injury: Evaluation of Clinical Signs, Mental Signs, and Quality of Life. Open Access Maced. J. Med. Sci. 7, 4399–4405 (2019).
- Vaquero, J. et al. Intrathecal administration of autologous mesenchymal stromal cells for spinal cord injury: Safety and efficacy of the 100/3 guideline. Cytotherapy 20, 806–819 (2018).
- Vaquero, J. et al. An approach to personalized cell therapy in chronic complete paraplegia: The Puerta de Hierro phase I/II clinical trial. Cytotherapy 18, 1025–1036 (2016).
- Yamazaki, K., Kawabori, M., Seki, T. & Houkin, K. Clinical Trials of Stem Cell Treatment for Spinal Cord Injury. Int. J. Mol. Sci. 21, 3994 (2020).
- Yang, Y. et al. Repeated subarachnoid administrations of allogeneic human umbilical cord mesenchymal stem cells for spinal cord injury: a phase 1/2 pilot study. Cytotherapy 23, 57–64 (2021).
- Zhao, H. et al. Is cell transplantation a reliable therapeutic strategy for spinal cord injury in clinical practice? A systematic review and meta-analysis from 22 clinical controlled trials. Eur. Spine J. 28, 1092–1112 (2019).
- Zhao, Y. et al. Study of the Diffusion Tensor Imaging for Preclinical Therapeutic Efficacy of Umbilical Cord Mesenchymal Stem Cell Transplantation in the Treatment of Spinal Cord Injury. Int. J. Gen. Med. Volume 14, 9721–9732 (2021).
- Mou, C. et al. Efficacy of mesenchymal stromal cells intraspinal transplantation for patients with different degrees of spinal cord injury: A systematic review and meta-analysis. Cytotherapy 25, 530–536 (2023).
- Yoon, S. H. et al. Complete Spinal Cord Injury Treatment Using Autologous Bone Marrow Cell Transplantation and Bone Marrow Stimulation with Granulocyte Macrophage-Colony Stimulating Factor: Phase I/II Clinical Trial. Stem Cells 25, 2066–2073 (2007).
- Bansal, H. et al. Autologous Bone Marrow-Derived Stem Cells in Spinal Cord Injury. J. Stem Cells 11, 51–61 (2016).
- Bhanot, Y. et al. Autologous mesenchymal stem cells in chronic spinal cord injury. Br. J. Neurosurg. 25, 516–522 (2011).
- Cheng, H. et al. Clinical observation of umbilical cord mesenchymal stem cell transplantation in treatment for sequelae of thoracolumbar spinal cord injury. J. Transl. Med. 12, 253 (2014).
- Dai, G. et al. Comparative analysis of curative effect of CT-guided stem cell transplantation and open surgical transplantation for sequelae of spinal cord injury. J. Transl. Med. 11, 315 (2013).
- Dai, G. et al. Transplantation of autologous bone marrow mesenchymal stem cells in the treatment of complete and chronic cervical spinal cord injury. Brain Res. 1533, 73–79 (2013).
- El-Kheir, W. A. et al. Autologous Bone Marrow-Derived Cell Therapy Combined with Physical Therapy Induces Functional Improvement in Chronic Spinal Cord Injury Patients. Cell Transplant. 23, 729–745 (2014).
- Bang, O. Y., Lee, J. S., Lee, P. H. & Lee, G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann. Neurol. 57, 874–882 (2005).
- Bhasin, A., Kumaran, S. S., Bhatia, R., Mohanty, S. & Srivastava, M. V. P. Letter: Safety and Feasibility of Autologous Mesenchymal Stem Cell Transplantation in Chronic Stroke in Indian patients. A four-year follow up. J. Stem Cells Regen. Med. 14, 59–60 (2018).
- Fuentes, B. et al. Short-Term Safety of Allogeneic Adipose-Tissue Derived Mesenchymal Stem Cells in Acute Ischemic Stroke (AMASCIS): A Phase II, Randomised, Double-Blind, Placebo-Controlled, Single-Centre, Pilot Clinical Trial. SSRN Electron. J. 1–35 (2019) doi:10.2139/ssrn.3420416.
- Hess, D. C. et al. Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 16, 360–368 (2017).
- Honmou, O. et al. Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain 134, 1790–1807 (2011).
- Jaillard, A. et al. Autologous Mesenchymal Stem Cells Improve Motor Recovery in Subacute Ischemic Stroke: a Randomized Clinical Trial. Transl. Stroke Res. 11, 910–923 (2020).
- Jiang, Y. et al. Feasibility of Delivering Mesenchymal Stem Cells via Catheter to the Proximal End of the Lesion Artery in Patients with Stroke in the Territory of the Middle Cerebral Artery. Cell Transplant. 22, 2291–2298 (2013).
- Kumar, A., Rawat, D. & Prasad, K. Stem cell therapy in ischemic stroke: A systematic review and meta-analysis of randomized controlled trials. Ann. Indian Acad. Neurol. 24, 164 (2021).
- Kawabori, M., Shichinohe, H., Kuroda, S. & Houkin, K. Clinical Trials of Stem Cell Therapy for Cerebral Ischemic Stroke. Int. J. Mol. Sci. 21, 7380 (2020).
- Kvistad, C. E. et al. Safety and Clinical Efficacy of Mesenchymal Stem Cell Treatment in Traumatic Spinal Cord Injury, Multiple Sclerosis and Ischemic Stroke – A Systematic Review and Meta-Analysis. Front. Neurol. 13, (2022).
- Lalu, M. M. et al. From the Lab to Patients: a Systematic Review and Meta-Analysis of Mesenchymal Stem Cell Therapy for Stroke. Transl. Stroke Res. 11, 345–364 (2020).
- Laskowitz, D. T. et al. Allogeneic Umbilical Cord Blood Infusion for Adults with Ischemic Stroke: Clinical Outcomes from a Phase I Safety Study. Stem Cells Transl. Med. 7, 521–529 (2018).
- Bhasin, A. et al. Stem cell therapy: A clinical trial of stroke. Clin. Neurol. Neurosurg. 115, 1003–1008 (2013).
- Lee, J. S. et al. A Long-Term Follow-Up Study of Intravenous Autologous Mesenchymal Stem Cell Transplantation in Patients With Ischemic Stroke. Stem Cells 28, 1099–1106 (2010).
- Lee, J. et al. Efficacy of Intravenous Mesenchymal Stem Cells for Motor Recovery After Ischemic Stroke: A Neuroimaging Study. Stroke 53, 20–28 (2022).
- Levy, M. L. et al. Phase I/II Study of Safety and Preliminary Efficacy of Intravenous Allogeneic Mesenchymal Stem Cells in Chronic Stroke. Stroke 50, 2835–2841 (2019).
- Li, Z. et al. Stem cell-based therapies for ischemic stroke: a systematic review and meta-analysis of clinical trials. Stem Cell Res. Ther. 11, 252 (2020).
- Nagpal, A. et al. Safety and effectiveness of stem cell therapies in early-phase clinical trials in stroke: a systematic review and meta-analysis. Stem Cell Res. Ther. 8, 191 (2017).
- Ouyang, Q. et al. Meta-Analysis of the Safety and Efficacy of Stem Cell Therapies for Ischemic Stroke in Preclinical and Clinical Studies. Stem Cells Dev. 28, 497–514 (2019).
- Paton, M. C. B. et al. Safety of allogeneic umbilical cord blood infusions for the treatment of neurological conditions: a systematic review of clinical studies. Cytotherapy 24, 2–9 (2022).
- Qiao, L.-Y. et al. A Two-Year Follow-Up Study of Cotransplantation with Neural Stem/Progenitor Cells and Mesenchymal Stromal Cells in Ischemic Stroke Patients. Cell Transplant. 23, 65–72 (2014).
- Sarmah, D. et al. Mesenchymal Stem Cell Therapy in Ischemic Stroke: A Meta‐analysis of Preclinical Studies. Clin. Pharmacol. Ther. 103, 990–998 (2018).
- Steinberg, G. K. et al. Two-year safety and clinical outcomes in chronic ischemic stroke patients after implantation of modified bone marrow-derived mesenchymal stem cells (SB623): a phase 1/2a study. J. Neurosurg. 131, 1462–1472 (2019).
- Bhasin, A. et al. Autologous Mesenchymal Stem Cells in Chronic Stroke. Cerebrovasc. Dis. Extra 1, 93–104 (2011).
- Steinberg, G. K. et al. Clinical Outcomes of Transplanted Modified Bone Marrow-Derived Mesenchymal Stem Cells in Stroke. Stroke 47, 1817–1824 (2016).
- Wang, K., Rong, L., Wei, X., Zhang, Q. & Xiao, L. The effectiveness of various cytotherapeutic strategies for the treatment of ischemic stroke: a Bayesian network meta-analysis of randomized controlled trials. Neurol. Sci. 41, 1705–1717 (2020).
- Zheng, H. et al. Mesenchymal Stem Cell Therapy in Stroke: A Systematic Review of Literature in Pre-Clinical and Clinical Research. Cell Transplant. 27, 1723–1730 (2018).
- de Celis-Ruiz, E. et al. Final Results of Allogeneic Adipose Tissue-Derived Mesenchymal Stem Cells in Acute Ischemic Stroke (AMASCIS): A Phase II, Randomized, Double-Blind, Placebo-Controlled, Single-Center, Pilot Clinical Trial. Cell Transplant. 31, 096368972210838 (2022).
- Permana, A. T. et al. Clinical outcome and safety of stem cell therapy for ischemic stroke: A systematic review and meta-analysis. Surg. Neurol. Int. 13, 206 (2022).
- Boncoraglio, G. B., Ranieri, M., Bersano, A., Parati, E. A. & Del Giovane, C. Stem Cell Transplantation for Ischemic Stroke. Stroke 51, E1–E2 (2020).
- Chen, L. et al. Multiple Cell Transplantation Based on an Intraparenchymal Approach for Patients with Chronic Phase Stroke. Cell Transplant. 22, 83–91 (2013).
- Chung, J.-W. et al. Efficacy and Safety of Intravenous Mesenchymal Stem Cells for Ischemic Stroke. Neurology 96, e1012–e1023 (2021).
- Cui, L., Golubczyk, D., Tolppanen, A., Boltze, J. & Jolkkonen, J. Cell therapy for ischemic stroke: Are differences in preclinical and clinical study design responsible for the translational loss of efficacy? Ann. Neurol. 86, 5–16 (2019).
- Detante, O., Moisan, A., Hommel, M. & Jaillard, A. Controlled clinical trials of cell therapy in stroke: Meta-analysis at six months after treatment. Int. J. Stroke 12, 748–751 (2017).
- Fang, J. et al. Autologous Endothelial Progenitor Cells Transplantation for Acute Ischemic Stroke: A 4-Year Follow-Up Study. Stem Cells Transl. Med. 8, 14–21 (2019).
- Wang, Z., Luo, Y., Chen, L. & Liang, W. Safety of neural stem cell transplantation in patients with severe traumatic brain injury. Exp. Ther. Med. 13, 3613–3618 (2017).
- Cramer, S. et al. Interim Analysis of the STEMTRA Trial: Safety and Dose-Dependent Benefit in Traumatic Brain Injury Patients. Arch. Phys. Med. Rehabil. 100, e22 (2019).
- Imai, H. et al. The international cooperative Phase II trial of modified stem cells (SB623) transplantation therapy for traumatic brain injury – Participation in a double-blind, controlled study (NCT02416492). J. Cereb. Blood Flow Metab. 39, 2–3 (2019).
- Kawabori, M. et al. Cell Therapy for Chronic TBI. Neurology 96, e1202–e1214 (2021).
- McCrea, M. A. et al. Determining minimally clinically important differences for outcome measures in patients with chronic motor deficits secondary to traumatic brain injury. Expert Rev. Neurother. 21, 1051–1058 (2021).
- Elkheir W., A. Allogenic Mesenchymal Stromal Cell Therapy for Type III Spinal Muscular Atrophy: Case Report. Am. J. Biosci. Bioeng. 3, 30 (2015).
- Villanova, M. & Bach, J. R. Allogeneic Mesenchymal Stem Cell Therapy Outcomes for Three Patients with Spinal Muscular Atrophy Type 1. Am. J. Phys. Med. Rehabil. 94, 410–415 (2015).
- Mohseni, R. et al. An open-label phase 1 clinical trial of the allogeneic side population adipose-derived mesenchymal stem cells in SMA type 1 patients. Neurol. Sci. 43, 399–410 (2022).
- Sharifzadeh, N. et al. Intrathecal autologous bone marrow stem cell therapy in children with autism: A randomized controlled trial. Asia. Pac. Psychiatry 13, e12445 (2021).
- Sun, J. M. et al. Infusion of human umbilical cord tissue mesenchymal stromal cells in children with autism spectrum disorder. Stem Cells Transl. Med. 9, 1137–1146 (2020).
- Lv, Y.-T. et al. Transplantation of human cord blood mononuclear cells and umbilical cord-derived mesenchymal stem cells in autism. J. Transl. Med. 11, 196 (2013).
- Wang, P. et al. Effects and safety of allogenic mesenchymal stem cell intravenous infusion in active ankylosing spondylitis patients who failed NSAIDs: a 20-week clinical trial. Cell Transplant. 23, 1293–1303 (2014).
- Li, A. et al. Infusion of umbilical cord mesenchymal stem cells alleviates symptoms of ankylosing spondylitis. Exp. Ther. Med. 14, 1538–1546 (2017).
- Wang, L. et al. Clinical Observation of Employment of Umbilical Cord Derived Mesenchymal Stem Cell for Juvenile Idiopathic Arthritis Therapy. Stem Cells Int. 2016, 1–7 (2016).
- Swart, J. F. et al. Bone-marrow derived mesenchymal stromal cells infusion in therapy refractory juvenile idiopathic arthritis patients. Rheumatology (Oxford). 58, 1812–1817 (2019).
- Álvaro-Gracia, J. M. et al. Intravenous administration of expanded allogeneic adipose-derived mesenchymal stem cells in refractory rheumatoid arthritis (Cx611): results of a multicentre, dose escalation, randomised, single-blind, placebo-controlled phase Ib/IIa clinical trial. Ann. Rheum. Dis. 76, 196–202 (2017).
- Yang, Y. et al. Serum IFN-γ levels predict the therapeutic effect of mesenchymal stem cell transplantation in active rheumatoid arthritis. J. Transl. Med. 16, 165 (2018).
- Wang, L. et al. Efficacy and Safety of Umbilical Cord Mesenchymal Stem Cell Therapy for Rheumatoid Arthritis Patients: A Prospective Phase I/II Study. Drug Des. Devel. Ther. Volume 13, 4331–4340 (2019).
- Ghoryani, M. et al. Amelioration of clinical symptoms of patients with refractory rheumatoid arthritis following treatment with autologous bone marrow-derived mesenchymal stem cells: A successful clinical trial in Iran. Biomed. Pharmacother. 109, 1834–1840 (2019).
- Xu, X. et al. Combination of human umbilical cord mesenchymal stem (stromal) cell transplantation with IFN-γ treatment synergistically improves the clinical outcomes of patients with rheumatoid arthritis. Ann. Rheum. Dis. 79, 1298–1304 (2020).
- Vij, R., Stebbings, K. A., Kim, H., Park, H. & Chang, D. Safety and efficacy of autologous, adipose-derived mesenchymal stem cells in patients with rheumatoid arthritis: a phase I/IIa, open-label, non-randomized pilot trial. Stem Cell Res. Ther. 13, 88 (2022).
- Liang, J. et al. Allogenic mesenchymal stem cells transplantation in refractory systemic lupus erythematosus: a pilot clinical study. Ann. Rheum. Dis. 69, 1423–1429 (2010).
- Sun, L., Wang, D., Liang, J., Zhang, H., Feng, X., Wang, H. Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum. 62, 2467–2475 (2010).
- Wang, D. et al. Double allogenic mesenchymal stem cells transplantations could not enhance therapeutic effect compared with single transplantation in systemic lupus erythematosus. Clin. Dev. Immunol. 2012, 273291 (2012).
- Wang, D. et al. Allogeneic mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus: 4 years of experience. Cell Transplant. 22, 2267–2277 (2013).
- Wang, D. et al. Umbilical cord mesenchymal stem cell transplantation in active and refractory systemic lupus erythematosus: a multicenter clinical study. Arthritis Res. Ther. 16, R79 (2014).
- Yang, G.-X. et al. [Therapeutic effects of umbilical cord mesenchymal stem cells transplantation on systemic lupus erythematosus]. Sichuan Da Xue Xue Bao. Yi Xue Ban 45, 338-341,350 (2014).
- Kamen, D. L. et al. Safety, immunological effects and clinical response in a phase I trial of umbilical cord mesenchymal stromal cells in patients with treatment refractory SLE. Lupus Sci. Med. 9, (2022).
- Al Demour, S. et al. Safety and Potential Therapeutic Effect of Two Intracavernous Autologous Bone Marrow Derived Mesenchymal Stem Cells injections in Diabetic Patients with Erectile Dysfunction: An Open Label Phase i Clinical Trial. Urol. Int. 101, 358–365 (2018).
- Al Demour, S. et al. Safety and Efficacy of 2 Intracavernous Injections of Allogeneic Wharton’s Jelly-Derived Mesenchymal Stem Cells in Diabetic Patients with Erectile Dysfunction: Phase 1/2 Clinical Trial. Urol. Int. 105, 935–943 (2021).
- Mirzaei, M. et al. The Effect of Intracavernosal Injection of Stem Cell in the Treatment of Erectile Dysfunction in Diabetic Patients: A Randomized Single-blinded Clinical Trial. Urol. J. 18, 675–681 (2021).
- You, D. et al. Safety of autologous bone marrow-derived mesenchymal stem cells in erectile dysfunction: an open-label phase 1 clinical trial. Cytotherapy 23, 931–938 (2021).
- Nguyen Thanh, L. et al. Can Autologous Adipose-Derived Mesenchymal Stem Cell Transplantation Improve Sexual Function in People with Sexual Functional Deficiency? Stem Cell Rev. Reports 17, 2153–2163 (2021).
- Protogerou, V. et al. Re: The Combined Use of Stem Cells and Platelet Lysate Plasma for the Treatment of Erectile Dysfunction: A Pilot Study – 6 Months Results. J. Urol. 204, 599–600 (2020).
- Levy, J. A., Marchand, M., Iorio, L., Cassini, W. & Zahalsky, M. P. Determining the feasibility of managing erectile dysfunction in humans with placental-derived stem cells. J. Am. Osteopath. Assoc. 116, e1–e5 (2016).
- Tan, J. et al. Autologous menstrual blood-derived stromal cells transplantation for severe Asherman’s syndrome. Hum. Reprod. 31, 2723–2729 (2016).
- Ding, L. et al. Transplantation of UC-MSCs on collagen scaffold activates follicles in dormant ovaries of POF patients with long history of infertility. Sci. China Life Sci. 61, 1554–1565 (2018).
- Tersoglio, A. E. et al. Regenerative therapy by endometrial mesenchymal stem cells in thin endometrium with repeated implantation failure. A novel strategy. JBRA Assist. Reprod. 24, 118–127 (2019).
- Zhang, Y. et al. Unresponsive thin endometrium caused by Asherman syndrome treated with umbilical cord mesenchymal stem cells on collagen scaffolds: a pilot study. Stem Cell Res. Ther. 12, 420 (2021).
- Chen, J., Huang, Q., Chen, W., Lin, S. & Shi, Q. Clinical Evaluation of Autologous and Allogeneic Stem Cell Therapy for Intrauterine Adhesions: A Systematic Review and Meta-Analysis. Front. Immunol. 13, 1–11 (2022).
- Cao, Y. et al. Allogeneic cell therapy using umbilical cord MSCs on collagen scaffolds for patients with recurrent uterine adhesion: A phase i clinical trial. Stem Cell Res. Ther. 9, 1–10 (2018).
- Santamaria, X. et al. Autologous cell therapy with CD133+ bone marrow-derived stem cells for refractory Asherman’s syndrome and endometrial atrophy: A pilot cohort study. Hum. Reprod. 31, 1087–1096 (2016).
- Nagori, C., Panchal, S. & Patel, H. Endometrial regeneration using autologous adult stem cells followed by conception by in vitro fertilization in a patient of severe Ashermans syndrome. J. Hum. Reprod. Sci. 4, 43–48 (2011).
- Ma, H. et al. Intrauterine transplantation of autologous menstrual blood stem cells increases endometrial thickness and pregnancy potential in patients with refractory intrauterine adhesion. J. Obstet. Gynaecol. Res. 46, 2347–2355 (2020).
- Kaczynski, J. B. & Rzepka, J. K. Endometrial regeneration in Asherman’s syndrome and endometrial atrophy using Wharton’s jelly-derived mesenchymal stem cells. Ginekol. Pol. 93, 904–909 (2022).